repair of 3 cm high ulnar nerve transection
A 26-year-old male sustained lacerations to his upper arm and forearm after falling through a glass window.
Author or contributor: Case courtesy of Dr. Bauback Safa
The Buncke Clinic
patient background
Patient information: 26-year-old male
Injury type: High ulnar nerve laceration
How injury occurred: Fell through a glass window
The patient demonstrated M4+ recovery of the FCU and significant improvement in grip strength, which is attributed to nerve regeneration through the Avance Nerve Grafts and not the more distal nerve transfer.
Avance Nerve Graft is an off-the-shelf processed human nerve allograft intended for the surgical repair of peripheral nerve discontinuities. Through a proprietary cleansing process, the nerve allograft preserves the inherent structure of the extracellular matrix (ECM) including the laminin rich endoneurial tubes of the native nerve tissue while clearing cellular and noncellular debris.
A 26-year-old man fell through a glass window and sustained lacerations to his upper arm and forearm and presented to the clinic one week after injury. On examination, there was no motor or sensory function in the distribution of the ulnar nerve, at either the forearm or the hand level.
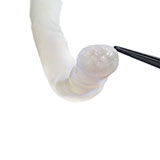
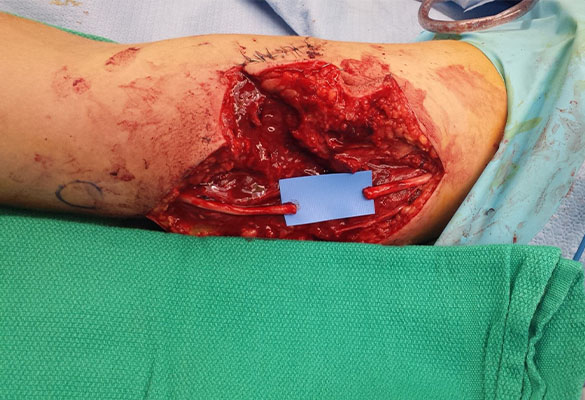
There was a complete transection of the ulnar nerve at the mid-upper arm. The ulnar nerve at the level of the forearm was found to be intact. Following nerve debridement, the resulting nerve gap was approximately 3 cm (Fig. 1).
High ulnar nerve injuries often result in poor recovery of intrinsic function. Furthermore, management of these large caliber mixed nerves, often larger than 5 mm in diameter, poses a significant clinical challenge as the surgeon must address multiple distal motor and sensory targets to restore function throughout the ulnar nerve distribution. Ensuring appropriate fascicular alignment during the repair will maximize the likelihood of meaningful recovery.
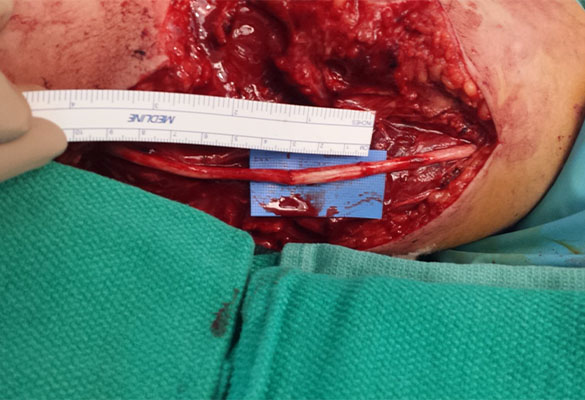
The proximal and distal ends of the ulnar nerve were debrided to healthy nerve and the resulting 3 cm gap was bridged with two Avance Nerve Grafts (3-4 mm diameter and 2-3 mm diameter) in a fascicular matching technique (Fig. 2). Distally, an end-to-end anterior interosseous nerve (AIN) to ulnar motor nerve transfer was performed to reinnervate the hand. A side-to-side tenodesis of the flexor digitorum profundus (FDP) tendons was also performed at the same time.
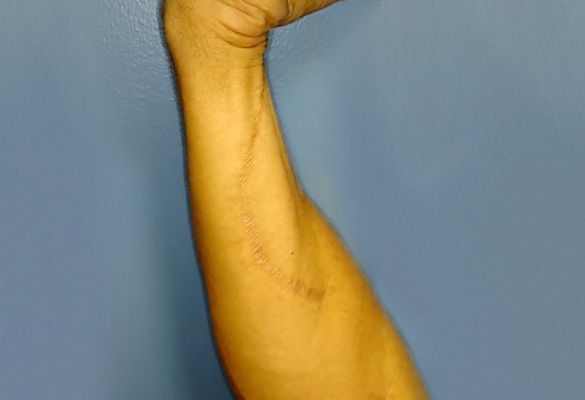
At one-year follow-up, the patient demonstrated excellent flexor carpi ulnaris (FCU) flexion against resistance (M4+ or greater), and his grip strength had improved significantly compared to his grip strength after the FDP tenodesis procedure. FCU function is supplied by the portion of the nerve that is proximal to the nerve transfer. Therefore, this motor recovery can be attributed to nerve regeneration through the Avance Nerve Grafts (Fig. 3).
Full transection of the ulnar nerve indicates that cross sprouting was not possible.
The off-the-shelf availability of Avance Nerve Grafts enabled a cable graft technique to adequately address the ulnar nerve’s diameter.
Though nerve and tendon transfers were performed as an adjunct to the allograft, FCU function is supplied by the portion of the nerve that is proximal to the nerve transfer.
Recovery observed is a result of nerve regeneration through the Avance Nerve Grafts.
82% meaningful recovery in sensory, mixed and motor nerve gaps in a multicenter study.1
Available in diameters up to 5 mm to enable fascicular matching.
Eliminates the need for donor site harvest and associated commorbidities.
Avance Nerve Graft
REGULATORY CLASSIFICATION: Avance Nerve Graft is processed and distributed in accordance with U.S. FDA requirements for Human Cellular and Tissue-Based Products (HCT/P) under 21 CFR Part 1271 regulations, U.S. State regulations and the guidelines of the American Association of Tissue Banks (AATB). Additionally, international regulations are followed as appropriate. Avance Nerve Graft is to be dispensed only by or on the order of a licensed physician.
INDICATIONS FOR USE: Avance Nerve Graft is processed nerve allograft (human) intended for the surgical repair of peripheral nerve discontinuities to support regeneration across the defect.
CONTRAINDICATIONS: Avance Nerve Graft is contraindicated for use in any patient in whom soft tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing or evidence of a current infection.
Disclaimer: The range of functional recovery can vary depending on the nature and extent of nerve damage, the timing of the repair, and the natural course of the patient’s recovery.