repair of 5 cm median and ulnar nerve gaps after circular saw injury
A 65-year-old male presented with a complex laceration after circular saw injury.
Author or contributor: Case courtesy of Dr. Bauback Safa
The Buncke Clinic
patient background
Patient information: 65-year-old male
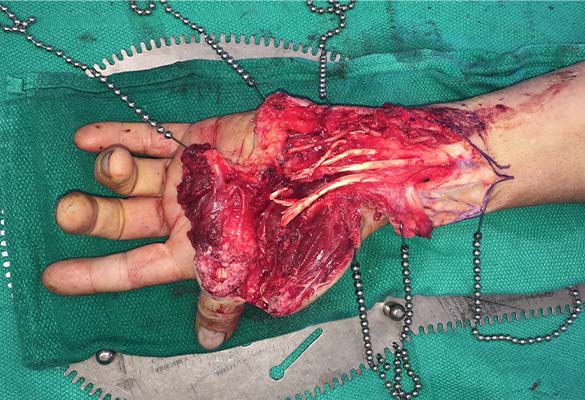
Injury type: Thumb devascularized and all major nerves, arteries and tendons (except for flexor digitorum profundus to the ring finger) were transected
How injury occurred: Circular saw injury
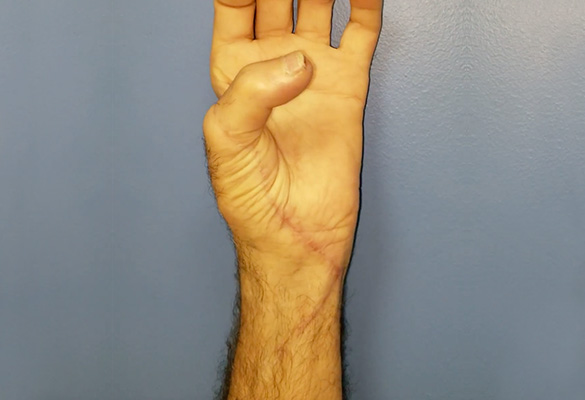
The patient demonstrated excellent intrinsic muscle tone and opposition of his thumb, confirming reinnervation of the median and ulnar nerve, through the Avance Nerve Graft.
Avance Nerve Graft is an off-the-shelf processed human nerve allograft intended for the surgical repair of peripheral nerve discontinuities. Through a proprietary cleansing process, the nerve allograft preserves the inherent structure of the extracellular matrix (ECM) including the laminin rich endoneurial tubes of the native nerve tissue while clearing cellular and non-cellular debris.
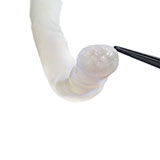
Axoguard Nerve Protector is the only surgical implant made from porcine small intestine submucosa ECM designed to protect injured nerves, reinforce the nerve reconstruction, and minimize soft tissue attachments.1 The patient’s cells remodel the material into the patient’s own tissue, similar to nerve epineurium.2
A 65-year-old man sustained a complex laceration to his left forearm and near amputation of the left thumb from a circular saw. The thumb was devascularized, and all major nerves, arteries and tendons (except for flexor digitorum profundus to ring finger) were transected (Fig. 1).
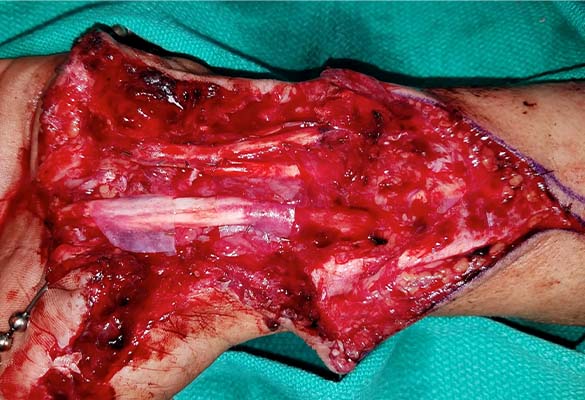
Both the median and ulnar nerves were larger than 5 mm in diameter and the resulting gap after debridement in both nerves was 5 cm.
The thumb was revascularized. All flexor tendons and the radial and ulnar arteries were repaired. The median nerve was repaired with two 5 cm long Avance Nerve Grafts used in a cable graft technique to ensure motor and sensory fascicular alignment. The ulnar nerve at the distal forearm was neurolysed and the motor and sensory components were repaired separately in a cable graft technique with two 5 cm long Avance Nerve Grafts. All coaptation sites were protected with Axoguard Nerve Protectors (Fig. 2).
At eight months follow-up, the patient had advancing Tinel’s to the metacarpophalangeal joint flexion creases of his fingers and nearly to the tip of the thumb. The patient demonstrated excellent opposition of his thumb confirming reinnervation of the recurrent branch of the median nerve and improved tone of his ulnar-innervated intrinsic muscles (Fig. 3). His extrinsic tendons glided well and he was able to make a composite fist.
Full transection of both median and ulnar nerve indicates that cross sprouting was not possible. Therefore, axon regeneration occurred through the Avance Nerve Graft.
Recovery observed is a result of regeneration through the Avance Nerve Grafts.
82% meaningful recovery in sensory, mixed and motor nerve gaps in a multi-center study.3
Available in diameters up to 5 mm to enable fascicular matching.
Eliminates the need for donor site harvest and associated commorbidities.
Avance Nerve Graft
REGULATORY CLASSIFICATION: Avance Nerve Graft is processed and distributed in accordance with U.S. FDA requirements for Human Cellular and Tissue-Based Products (HCT/P) under 21 CFR Part 1271 regulations, U.S. State regulations and the guidelines of the American Association of Tissue Banks (AATB). Additionally, international regulations are followed as appropriate. Avance Nerve Graft is to be dispensed only by or on the order of a licensed physician.
INDICATIONS FOR USE: Avance Nerve Graft is processed nerve allograft (human) intended for the surgical repair of peripheral nerve discontinuities to support regeneration across the defect.
CONTRAINDICATIONS: Avance Nerve Graft is contraindicated for use in any patient in whom soft tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing or evidence of a current infection.
Disclaimer:
The range of functional recovery can vary depending on the nature and extent of nerve damage, the timing of the repair, and the natural course of the patient’s recovery.
Axoguard Nerve Protector
INDICATIONS FOR USE: The Axoguard Nerve Protector is indicated for the repair of peripheral nerve injuries where there is no gap. The device is supplied sterile and is intended for one-time use.
CONTRAINDICATIONS: This device is derived from a porcine source and should not be used for patients with known sensitivity to porcine material.